Bio & Pharma
Celltrion ships initial supply of Zymfentra to US
The S.Korean company's autoimmune disease treatment is scheduled to be available in the local market in March
By Feb 29, 2024 (Gmt+09:00)
1
Min read
Most Read
MBK’s Korea Zinc takeover attempt to spur search for white knights


Korea Zinc, MBK face proxy war for zinc smelter


Korea Zinc shares skyrocket after buybacks in tender offer


Lotte to liquidate rubber JV in Malaysia, sell overseas assets for $1 bn


Samsung to unveil 400-layer bonding vertical NAND for AI servers by 2026


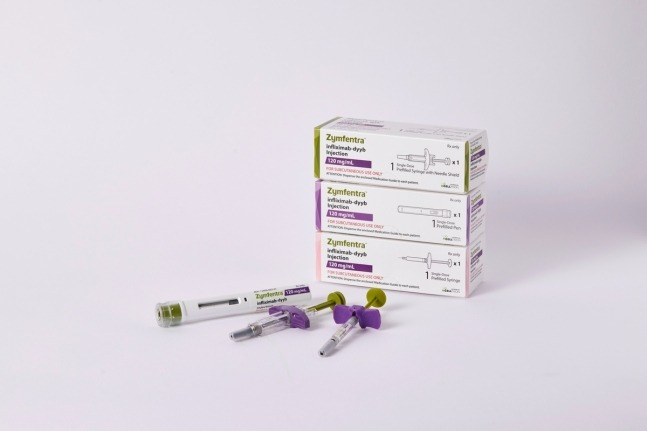
South Korea's Celltrion Inc. announced on Wednesday that it shipped the first supply of its subcutaneous injection type of the autoimmune disease treatment Remsima SC (active ingredient infliximab, American name Zymfentra) to the US.
The completed product shipped today will go through local logistics processes, including import clearance, transportation, and wholesale warehousing, and is expected to be available in the market from mid-March.
Celltrion plans to ship all three batches of the initial supply of Zymfentra from Wednesday until early March.
Zymfentra was developed by changing the existing intravenous injection type autoimmune disease treatment Remsima into a subcutaneous injection type, and it obtained new drug approval (NDA) from the US Food and Drug Administration (FDA) in October last year.
The product has obtained NDA in more than 50 countries worldwide, including Europe and Canada.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com
More to Read
-

-
-
 Bio & PharmaCelltrion, WuXi XDC to develop new antibody-drug conjugates
Bio & PharmaCelltrion, WuXi XDC to develop new antibody-drug conjugatesJan 24, 2024 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion to export three anticancer drugs to Europe
Bio & PharmaCelltrion to export three anticancer drugs to EuropeJan 18, 2024 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Holdings eyes Nasdaq IPO by early 2025: chairman
Bio & PharmaCelltrion Holdings eyes Nasdaq IPO by early 2025: chairmanJan 16, 2024 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


