Bio & Pharma
Celltrion's Zymfentra joins US insurance-covered drug lists
The new biosimilar secures access to 80% of the US insurance-covered drug market
By Aug 06, 2024 (Gmt+09:00)
1
Min read
Most Read
When in S. Korea, it’s a ritual: Foreigners make stops at CU, GS25, 7-Eleven


Maybe Happy Ending: A robot love story that rewrote Broadway playbook


NPS yet to schedule external manager selection; PE firms’ fundraising woes deepen


Samsung steps up AR race with advanced microdisplay for smart glasses


Seoul appeal: Korean art captivates Indonesia’s affluent connoisseurs


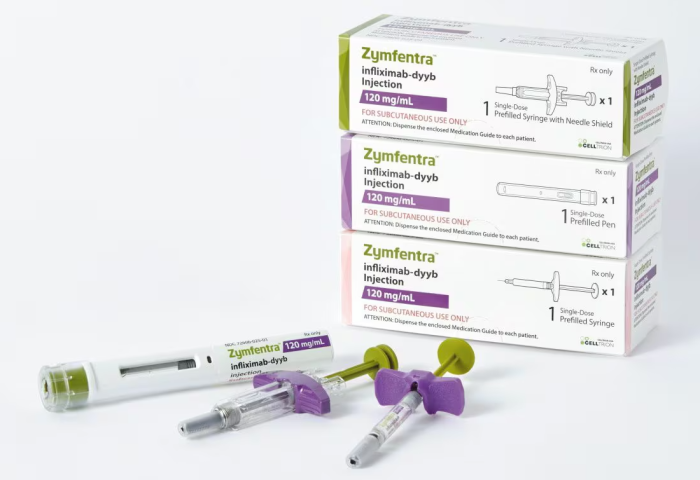
South Korean biosimilar maker Celltrion Inc. said on Monday that its subcutaneous injection formulation for autoimmune diseases, Zymfentra (infliximab), has joined the insurance-covered drug lists by three major US Prescription Benefit Managers (PBMs) after its US launch in March.
The three PBMs are Express Scripts, OptumRx and CVS Health. They control about 80% of prescription drugs covered by insurance in the US. They act as intermediaries between pharmacies, drug manufacturers and insurance companies.
The contracts were signed between April and early this month.
With one of the PBMs, Celltrion has secured a public insurance contract and is negotiating to get the biosimilar covered by private insurance plans as well.
Zymfentra, a self-administered version of Celltrion's intravenous biosimilar "Remsima," was approved as a new drug in the US last October.
It treats inflammatory bowel diseases like ulcerative colitis and Crohn's disease.
According to IQVIA, the inflammatory bowel disease treatment market in the US is estimated at 14 trillion won ($10.2 billion).
Celltrion aims to supply Zymfentra to 150,000 inflammatory bowel disease patients in the US by next year, or half of some 300,000 patients suffering from the disease in the country, according to Celltrion founder and Chairman Seo Jung-jin.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com
More to Read
-
 Bio & PharmaCelltrion eyes M&A in Europe, $3.3 bn Zymfentra sales: chairman
Bio & PharmaCelltrion eyes M&A in Europe, $3.3 bn Zymfentra sales: chairmanMay 23, 2024 (Gmt+09:00)
4 Min read -
 Bio & PharmaCelltrion'a autoimmune disease drug Zymfentra makes US debut
Bio & PharmaCelltrion'a autoimmune disease drug Zymfentra makes US debutMar 18, 2024 (Gmt+09:00)
1 Min read -
Comment 0
LOG IN


