JLK gets FDA OK for prostate cancer diagnosis AI solution
It will boost expanding into the US market with its Medihub Prostate can MR images and prostate-specific antigen
By Jun 25, 2024 (Gmt+09:00)
Samsung steps up AR race with advanced microdisplay for smart glasses


When in S. Korea, it’s a ritual: Foreigners make stops at CU, GS25, 7-Eleven


Maybe Happy Ending: A robot love story that rewrote Broadway playbook


NPS yet to schedule external manager selection; PE firms’ fundraising woes deepen


US auto parts tariffs take effect; Korea avoids heavy hit


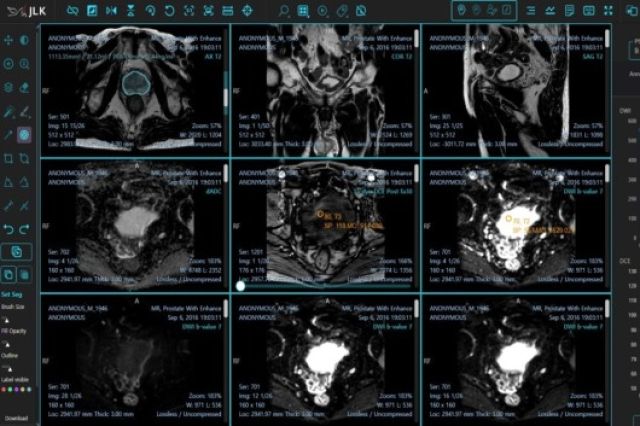
South Korea’s JLK Inc., an AI-based diagnosis solution and platform maker, announced on Monday that it got approval from the US Food and Drug Administration (FDA) for its prostate cancer diagnostic solution Medihub Prostate.
The solution aids prostate cancer diagnosis by analyzing prostate magnetic resonance (MR) images and assessing prostate-specific antigen (PSA) levels.
Prostate cancer is the most common cancer among men in the US, with a latent prostate cancer occurrence risk of up to 40%.
"Based on the FDA approval of MEDIHUB Prostate, we plan to pursue entry into the US market more aggressively," JLK's CEO Kim Dong-min said.
"We intend to sequentially apply for FDA approval for three additional AI solutions between August and October."
Write to Jeong Min Nam at peux@hankyung.com
-

-
 Bio & PharmaKorea's JLK targets AI-based stroke care market in US
Bio & PharmaKorea's JLK targets AI-based stroke care market in USJan 10, 2024 (Gmt+09:00)
2 Min read -