Bio & Pharma
S.Korea’s Lunit installs breast cancer AI diagnostic tools at 200 US hospitals
The Seoul-based medtech firm makes rapid inroads into the US healthcare market, bolstered by the Volpara acquisition
By 6 HOURS AGO
2
Min read
Most Read
In China’s waterway city Hangzhou, K-beauty redefines ‘shuiguang'


South Korea’s Rznomics inks $1.3 bn out-licensing deal with Eli Lilly


When in S. Korea, it’s a ritual: Foreigners make stops at CU, GS25, 7-Eleven


Mubadala, Goldman Sachs to invest $700 mn in Kakao Mobility


Samsung steps up AR race with advanced microdisplay for smart glasses


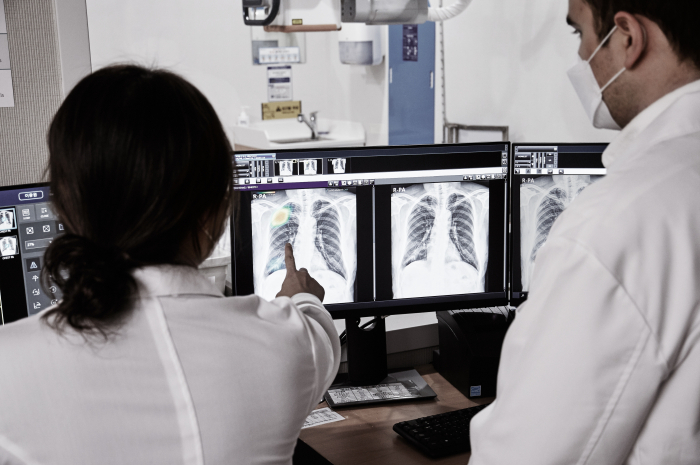
Lunit Inc., a South Korean AI-powered cancer diagnostics company, said on Monday that more than 200 healthcare institutions across the US have now adopted its breast cancer diagnostic equipment – a key milestone one year after acquiring New Zealand-based breast imaging analytics firm Volpara Health Technologies Ltd.
Before the May 2024 acquisition, Lunit had no US-based deployments of its AI-powered breast cancer diagnosis devices.
The company credited Volpara’s established commercial network in the US for the swift rollout, allowing Lunit to integrate its technology into major institutions such as SimonMed Imaging – the country’s largest private radiology provider – and UC Davis Health, a prominent academic hospital network on the West Coast.
Lunit and Volpara together support the analysis of over 1 million mammography examinations annually in North America.
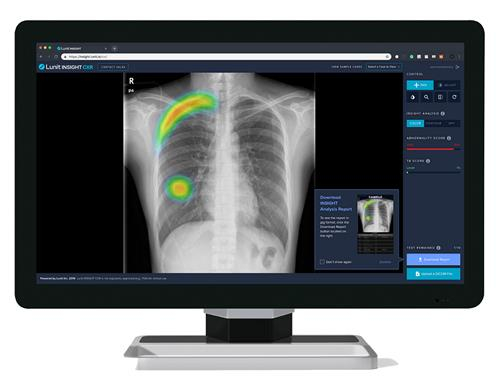
Lunit offers two AI-powered imaging analysis tools: Lunit Insight MMG for interpreting X-ray mammography images and Lunit Insight DBT for digital breast tomosynthesis scans, or 3D mammography.
DISTINGUISHED BY ACCURACY, VERSATILITY
The company said its AI diagnostic devices are distinguished by their high detection accuracy and versatility in handling both two- and three-dimensional imaging data.

In 2023, Lunit obtained approval for its AI cancer diagnosis programs – Lunit Insight DBT, Lunit Insight MMG and AI triage device Lunit Insight Triage – from the US Food and Drug Administration (FDA).
“Our differentiated AI technology is being recognized in the US market for its ability to detect suspicious lesions with precision,” said a Lunit official.
Looking beyond diagnostics, Lunit is positioning itself as a comprehensive cancer care platform.
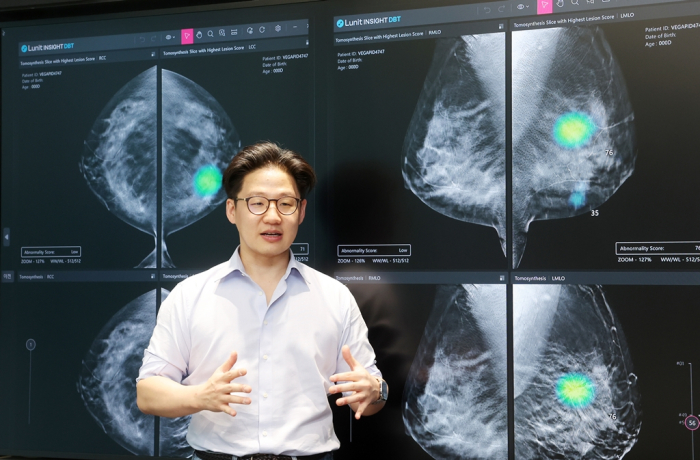
At the Radiological Society of North America (RSNA) 2024 conference in December, the company unveiled Lunit Insight Risk – a tool designed to predict the likelihood of developing breast cancer within one to five years.
Lunit plans to seek FDA approval for the new risk assessment system in the second half of this year, paving the way for its global rollout starting in North America.
Lunit, listed on Korea’s tech-heavy Kosdaq, last November partnered with global biopharma giant AstraZeneca plc. to jointly enter the AI-powered lung cancer diagnostics market.
Write to Hyun-Ah Oh at 5hyun@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Bio & PharmaKorea’s Lunit in AI-powered lung cancer diagnostics deal with AstraZeneca
Bio & PharmaKorea’s Lunit in AI-powered lung cancer diagnostics deal with AstraZenecaNov 18, 2024 (Gmt+09:00)
2 Min read -
 Artificial intelligenceLunit eyes developing markets with AI cancer diagnostics
Artificial intelligenceLunit eyes developing markets with AI cancer diagnosticsJul 09, 2024 (Gmt+09:00)
4 Min read -
 Mergers & AcquisitionsLunit buys breast cancer detection AI firm Volpara Health
Mergers & AcquisitionsLunit buys breast cancer detection AI firm Volpara HealthMay 22, 2024 (Gmt+09:00)
2 Min read -

Comment 0
LOG IN


