Yuhan’s lung cancer drug clears FDA hurdle for global debut
The medicine is expected to become a blockbuster drug with annual sales of over 1 trillion won
By Aug 21, 2024 (Gmt+09:00)
Samsung steps up AR race with advanced microdisplay for smart glasses


When in S. Korea, it’s a ritual: Foreigners make stops at CU, GS25, 7-Eleven


Maybe Happy Ending: A robot love story that rewrote Broadway playbook


NPS yet to schedule external manager selection; PE firms’ fundraising woes deepen


US auto parts tariffs take effect; Korea avoids heavy hit


A new lung cancer treatment developed by South Korea’s Yuhan Corp. has secured approval from the US Food and Drug Administration (FDA) as a combination therapy with a Johnson & Johnson’s (J&J) antibiotic, the US pharmaceutical company said on Tuesday.
Lazertinib, sold by Yuhan under the brand name Leclaza in South Korea, and J&J’s Rybrevant got the nod from the FDA as a first-line combination treatment for adult patients with advanced non-small cell lung cancer (NSCLC), based on their phase 3 clinical trial results, according to J&J.
With the approval, Lazertinib became the first antibiotic drug developed by a South Korean company and is poised to become the country's first blockbuster drug with annual sales of over 1 trillion won ($750 million).
In 2018, Yuhan signed a licensing-out agreement of up to $1.25 billion for Lazertinib with Janssen Biotech Inc. of J&J for clinical trials and global approval. It is an oral, third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI).
In 2015, Yuhan purchased the Lazertinib substance license from Genosco, a Boston-based R&D arm of Oscotec Inc. in the substance development phase.
Lazertinib is also called Lazcluze.
“With this milestone, Rybrevant plus Lazcluze becomes the first and only multitargeted, chemotherapy-free combination regimen with demonstrated superiority versus Osimertinib approved for first-line treatment of patients with EGFR-mutated NSCLC,” J&J said in a statement.
Osimertinib, developed by AstraZeneca, is sold under the brand name Tagrisso. It dominates the EGFR-mutated NSCLC drug market.
NSCLC makes up 80 to 85 percent of lung cancers, according to the American Cancer Society.
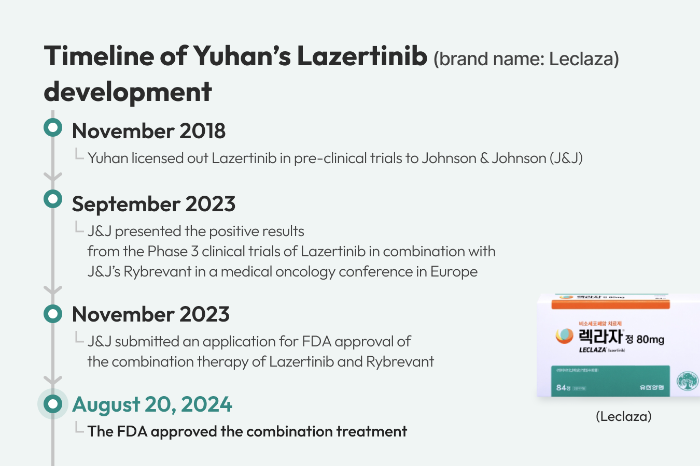
In clinical trials, the Rybrevant and Lazertinib combination therapy reduced the risk of disease progression or death by 30 percent versus Osimertinib with a nine-month-longer progression-free survival period, J&J added.
But it changed its strategy late last year to directly purchase innovative drug candidates and develop other blockbuster medicines. Out of its 33 drug candidates in the pipeline, 16 are from outside companies.

FINANCIAL TARGETS
It targets about a ninefold jump in operating profit to 500 billion won by 2026, from 56.8 billion won in 2023.
BIOSIMILAR MARKET
South Korea’s innovative drug development sector is in its infancy. But it has built its strong presence in the biosimilar market, developing an average of 1.5 new biosimilars annually, led by Celltrion Inc.
Celltrion's Remsima, referencing Remicade, was approved by Europe as the world's first antibody biosimilar in 2013 and got the nod from the FDA in 2016
Of 56 biosimilars approved by the FDA in the first half of this year, South Korea had 12 to rank second in the market after the US with 24.
(Updated with comments from Yuhan CEO Cho Wook-je)
Write to Yu-Rim Kim and Young-Ae Lee at youforest@hankyung.com
Yeonhee Kim edited this article.
-
 Bio & PharmaCelltrion's Remsima SC breaks 20% market share in Europe
Bio & PharmaCelltrion's Remsima SC breaks 20% market share in EuropeJul 17, 2024 (Gmt+09:00)
1 Min read -

-
 Bio & PharmaKorea’s Celltrion, SK Biopharmaceuticals benefit from direct US sales
Bio & PharmaKorea’s Celltrion, SK Biopharmaceuticals benefit from direct US salesApr 16, 2024 (Gmt+09:00)
3 Min read -
 Bio & PharmaJ&J seeks US, European approval for drug developed with Yuhan
Bio & PharmaJ&J seeks US, European approval for drug developed with YuhanDec 22, 2023 (Gmt+09:00)
2 Min read -
 EarningsCelltrion enjoys record earnings on healthy biosimilar biz
EarningsCelltrion enjoys record earnings on healthy biosimilar bizNov 07, 2023 (Gmt+09:00)
3 Min read -
 Bio & PharmaGI Innovation seeks to license allergy therapy to Japan in 2023
Bio & PharmaGI Innovation seeks to license allergy therapy to Japan in 2023Jul 17, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaNovel medicines poised to be Korea’s next mainstay export
Bio & PharmaNovel medicines poised to be Korea’s next mainstay exportFeb 27, 2023 (Gmt+09:00)
3 Min read -