LG Chem launches Synovian in China
The company sells its homegrown new treatment for osteoarthritis through local partner Yifan Pharmaceutical
By Jul 03, 2024 (Gmt+09:00)
Samsung steps up AR race with advanced microdisplay for smart glasses


When in S. Korea, it’s a ritual: Foreigners make stops at CU, GS25, 7-Eleven


Maybe Happy Ending: A robot love story that rewrote Broadway playbook


NPS yet to schedule external manager selection; PE firms’ fundraising woes deepen


US auto parts tariffs take effect; Korea avoids heavy hit


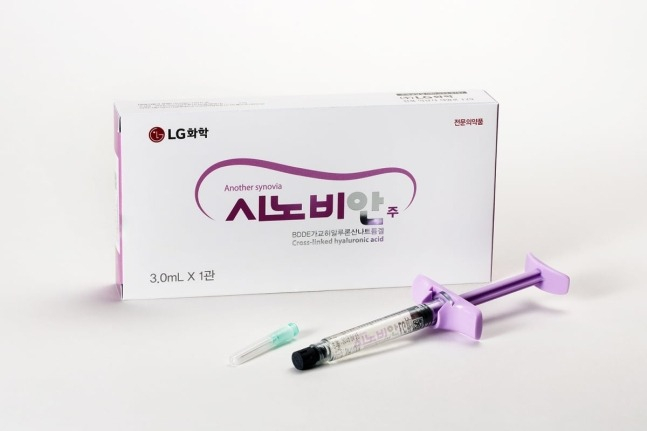
South Korea's LG Chem Ltd. announced on Wednesday that it launched its homegrown new drug for osteoarthritis Synovian (marketed in China as Hyruan ONE) in China with its local partner company Yifan Pharmaceutical.
Yifan Pharmaceutical, established in 2000 and based in Hangzhou, China, is a comprehensive pharmaceutical company with a broad business portfolio that includes anti-inflammatory, anti-cancer, and endocrine/metabolic disease treatments.
The Chinese company has a dense sales network across China, employing over 6,000 people and recording annual sales of $800 million.
Yifan is recognized for its strong R&D and commercialization capabilities, having been listed among "China's Top-100 Innovative Pharmaceutical Companies" (CPIE 100) in 2022.
Synovian was developed by LG Chem using its technology. It is a new drug for knee osteoarthritis treatment, launched domestically in 2014, containing cross-linked hyaluronic acid (HA) that offers similar therapeutic effects to multi-injection formulations with a single administration.
Clinical phase 3 trials on Chinese patients with knee osteoarthritis showed that Synovian's pain reduction and joint function improvement effects its safety, were similar to those of multi-injection HA products.
LG Chem partnered with Yifan Pharmaceutical and prepared for the business in stages.
Yifan began local phase 3 clinical trials in August 2019, applied for marketing approval in December 2021, and received the approval in April 2023.
After a year of negotiations with the Chinese government, Synovian was listed on the National Reimbursement Drug List (NRDL), signaling a successful market entry.
According to pharmaceutical market research firm IQVIA, the Chinese market for osteoarthritis HA injectables is worth $144 million, making it the third largest in the world after the US and Japan.
Synovian is the only single-injection formulation in the Chinese market, offering a clear competitive advantage over the commonly prescribed five-injection formulation (one injection per week for five weeks).
LG Chem conducted a treatment preference survey among Chinese doctors, and a significant majority (87%) of respondents expressed a positive inclination toward the single-injection formulation, citing advantages such as lower infection risk and convenience for patients from remote areas.
LG Chem focuses on enhancing business capabilities by regularly running an onboarding program to help Yifan quickly absorb its domestic business experience with Synovian.
The company also plans to support Yifan's local sales and marketing activities by developing academic content for physicians and promoting academic exchanges between South Korea and China.
Write to Young-ae Lee at 0ae@hankyung.com
-
 Chemical IndustryLG Chem, KCC partner to develop eco-friendly paint
Chemical IndustryLG Chem, KCC partner to develop eco-friendly paintMay 27, 2024 (Gmt+09:00)
1 Min read -
 Chemical IndustryLG Chem, Saudi’s Alkhorayef join hands for seawater project
Chemical IndustryLG Chem, Saudi’s Alkhorayef join hands for seawater projectMay 01, 2024 (Gmt+09:00)
2 Min read -
 EarningsLG Chem displays bright outlook after loss in first quarter
EarningsLG Chem displays bright outlook after loss in first quarterApr 30, 2024 (Gmt+09:00)
3 Min read -
 Chemical IndustryLG Chem to supply switchable glass films to Webasto
Chemical IndustryLG Chem to supply switchable glass films to WebastoApr 29, 2024 (Gmt+09:00)
2 Min read -
 BatteriesLG Chem, Korea Zinc JV to mass-produce EV battery materials
BatteriesLG Chem, Korea Zinc JV to mass-produce EV battery materialsApr 17, 2024 (Gmt+09:00)
2 Min read


